The EU has approved a new HIV drug
HIV infections are currently on the decline again in many parts of the world, and the WHO has often made the optimistic prediction that HIV could be eradicated as early as 2030.
However, it should not be forgotten that many people still become infected or suffer from the effects of HIV.
Although medical science has been able to make many advances in the treatment of HIV over the decades, sufferers usually have to take various preparations every day in order to be able to lead a life - ideally - free of symptoms. There is still no real cure.
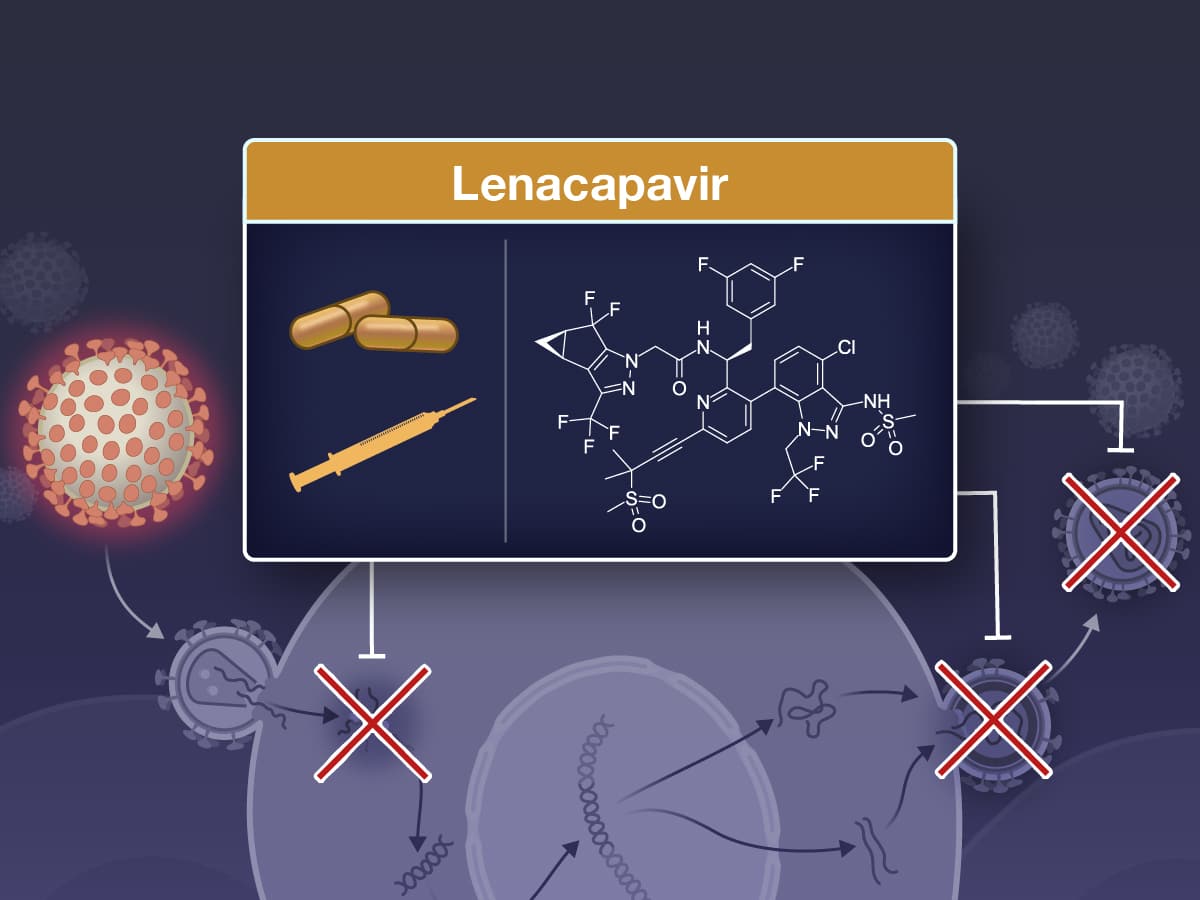
HIV drug lenacapavir (Sunlenca®, Gilead) is approved - but what changes?
The fact that new HIV drugs are being approved to combat various diseases does not yet represent a real revolution. Lenacapavir (Sunlenca®, Gilead), however, is a long-acting drug that belongs to the capsid inhibitors. The special feature: This drug is to be taken orally in the first step and, after a certain time, administered as an injection only twice a year.
Treatment would therefore be much more "comfortable" than is currently the case. People suffering from HIV would thus have the opportunity to regain a large part of their quality of life. After all, research is not only about curing people, but also about simplifying things, while at the same time being naturally effective and improving the lives of those affected. Above all, there is a higher life expectancy with the claim to also enjoy the pleasure of existence.
What is the role of the intake interval?
There is no question that the new HIV drug lenacapavir (Sunlenca®, Gilead) is so attractive simply because it has to be taken or injected comparatively rarely. It even outperforms the previous frontrunners Cabotegravir (Vocabria®) and Rilpivirin (Rekambys®). This is because the newly approved drug only has to be taken orally for a period of two weeks. After that, an injection is administered every six months.
The approval benefits adults who are not eligible for most of the other drugs that have been approved for HIV. The drug was developed in the USA and has managed to be present in the media comparatively quickly. One of the reasons for this is that it is so convenient to take and treat.
Currently, no cross-resistance to other agents is known (yet?). Most patients who have already been exposed to the treatment have tolerated lenacapavir (Sunlenca®, Gilead) well. If all factors were right, no viral load could be detected after about six months.
One admission and a whole lot of hope
The diagnosis of "AIDS" largely turns the lives of those affected upside down. In addition to having to come to terms with the fact that one's own life expectancy may dwindle, there is also the (often) costly therapy involved.
The daily intake of medication, which is not always well tolerated, can often lead to depression, among other things. If the new HIV drug continues to gain acceptance, it is certainly no exaggeration to speak of a kind of "revolution. After all, it would significantly simplify the treatment of AIDS.
The new options would be much easier to integrate into everyday life, and those affected would also not be constantly reminded that they are carrying the HI virus. However, the approval of the drug also proves, among other things, how intensively research has worked on one (or more) HIV drug(s) in recent years.
The fight against AIDS is not over yet. However, the WHO's goal of eradicating the disease by 2030 is a little closer.